IPS Implants® Mandible Reconstruction
Jaw defects due to trauma, tumors, infections, or extreme atrophy affect the quality of life of patients - both physiologically and psychologically.
Jaw defects due to trauma, tumors, infections, or extreme atrophy affect the quality of life of patients - both physiologically and psychologically.
Approximately 20% of all IPS® cases are IPS Implants® Mandible Reconstruction cases. Resection of a tumor in the mandible and subsequent reconstruction with autogenous bone is a very demanding operation. It requires a 1:1 transfer of the donor site to the mandible to obtain a perfectly fitting bone graft. Therefore, custom fabrication using computer-based planning is one of the best and most precise ways.
To date, thousands of implants have been successfully placed. We offer all types of implants and accessories (marking guides or models) for jaw reconstruction for the best possible individual treatment of complex defect situations.
The planning process for an IPS® Mandible Reconstruction implant takes place through simple and efficient interaction between the treating clinician and our IPS® engineers via IPS Gate®.
Thanks to a wide range of planning options, bone thickness, implants including screw positions and the postoperative situation can be simulated in detail. The consideration of the vascular supply and the planning of the resulting vascular pedicle is decisive for the success of the transplantation.
Planning, production, and dispatch are all carried out in a single system, eliminating the need for coordination between several service providers. For you, this means maximum mobility, flexibility, and functionality.
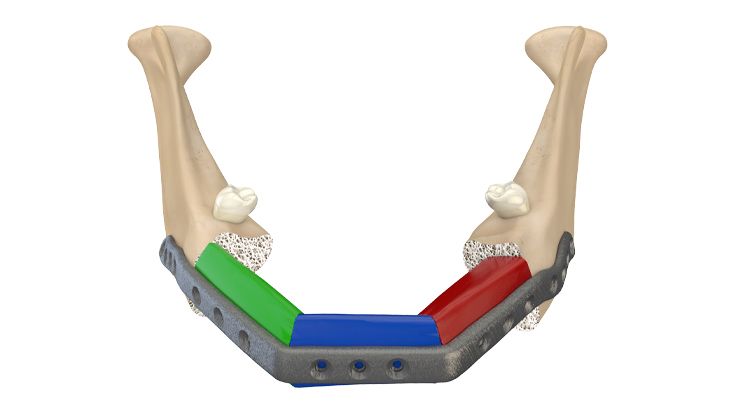
Using additive manufacturing, an IPS® implant made of high-strength titanium alloy is then fabricated - based on the individual case planning. The implants are dimensionally stable during insertion and are already checked at the factory for optimal three-dimensional accuracy of fit using a custom-made model.

Patient-specific drill and marking guides allow the position and screw holes of the IPS Implants® to be determined precisely, thus ensuring a smooth transfer of the virtual planning to the operating room. Integrated steel sleeves allow direct drilling without additional drill sleeves. If desired, the drill and marking guides can be made of additively manufactured titanium and, in this case, can also be used as a sawing guide.
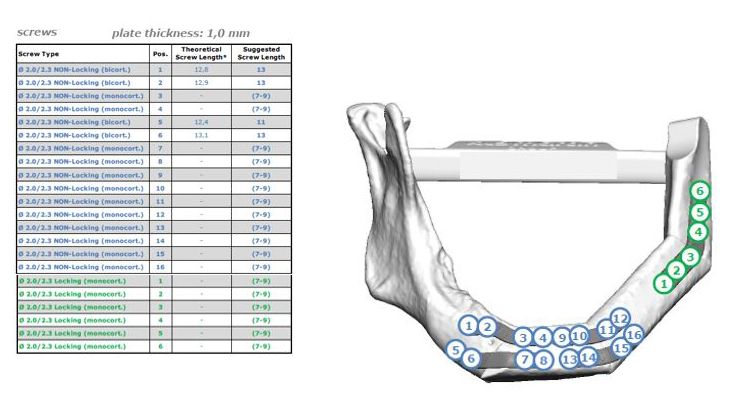
In addition, you will receive a screw table coordinated with you for transplant planning. This table specifies which screw hole must be filled with which screw to further optimize the surgical procedure.
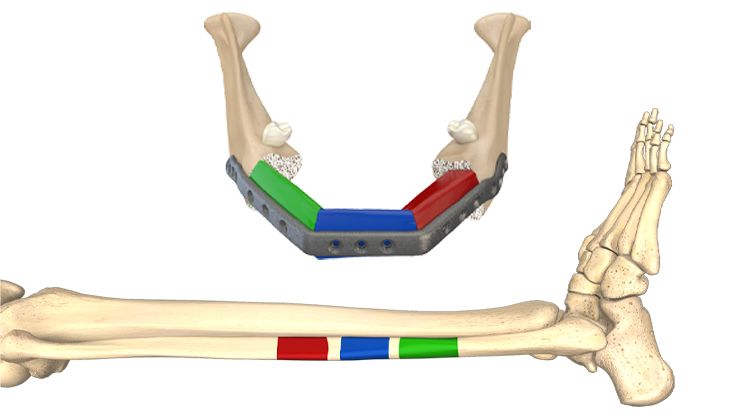
1. Preliminary: virtual planning
For case creation, patient data and other case-related information are uploaded to the web-based platform IPS Gate®.
Based on the information and requests you provide, the IPS® developer prepares the case planning.
First, the resection margins are defined. Virtually, the donor region is projected onto the recipient region and the graft is optimized for its best possible esthetic and functional restoration.
Next, drill and marking guides and a case-specific optimized implant are generated. Finally, you release the design for production and the products are shipped.
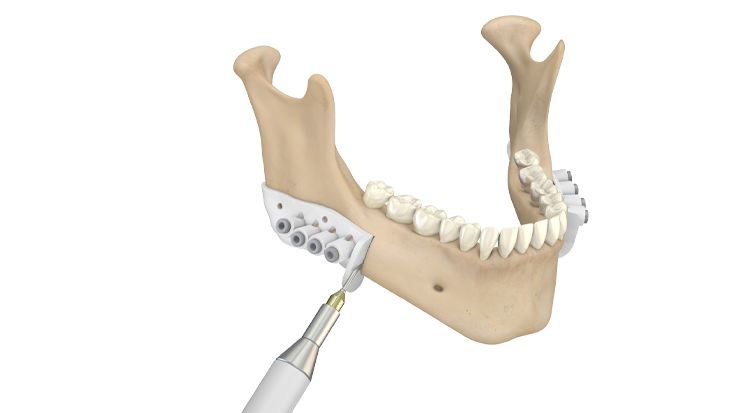
2. In the operating room: resection of the mandible
After preparation of the mandible, the drill and marking guides, which also reflect the cutting angle, are attached to the mandible with osteosynthesis screws. The small holes in the drill and marking guides are used to attach them to the mandible.
The resection lines are then marked (in the illustration with a piezo device). The screw holes of the implant are predrilled through the large holes in the drill and marking guides. The holes are provided with steel sleeves through which targeted drilling can be performed without inserting additional drill sleeves.
The drill and marking guide is removed and the resection is performed along the marked line.

3. In the operating room: resection of the fibula
The fibula is prepared simultaneously to the mandible. Comparable to the recipient region, the resection is performed in the donor region with the help of the drill and marking guide, which is fixed to the fibula with osteosynthesis screws.
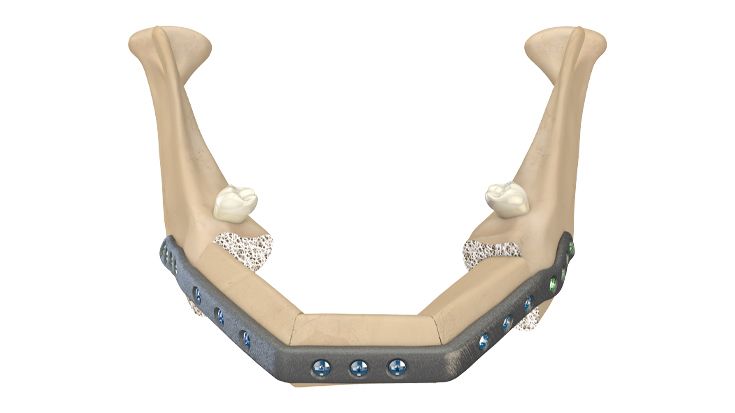
4. In the operating room: fixation of the implant
The IPS® plate is fixed in the pre-drilled holes on the mandible with the planned osteosynthesis screws. The graft parts are inserted and the anastomosis is performed. The graft parts are then fixed with osteosynthesis screws. As an alternative to the above procedure, the bone graft parts can also be attached to the IPS® plate in advance and then fixed to the mandible together with the plate.
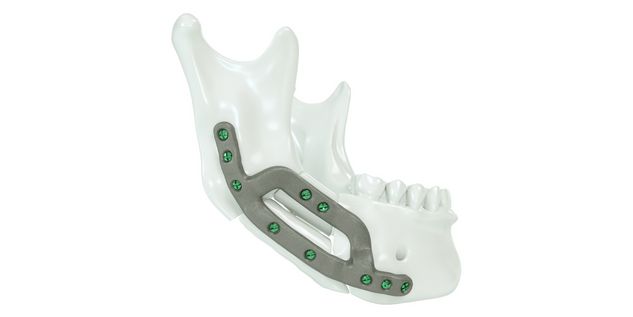
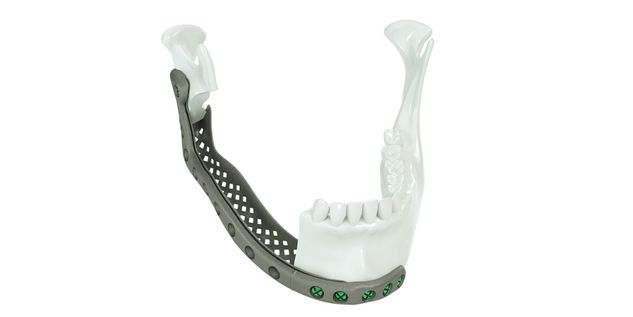
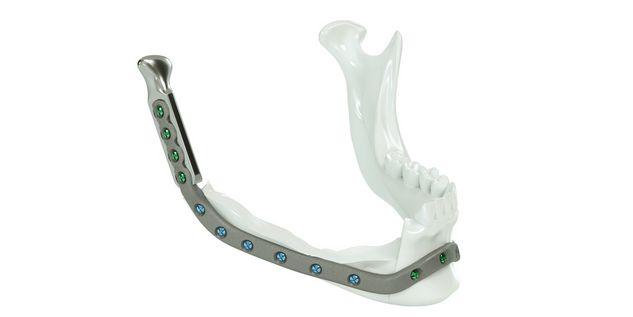
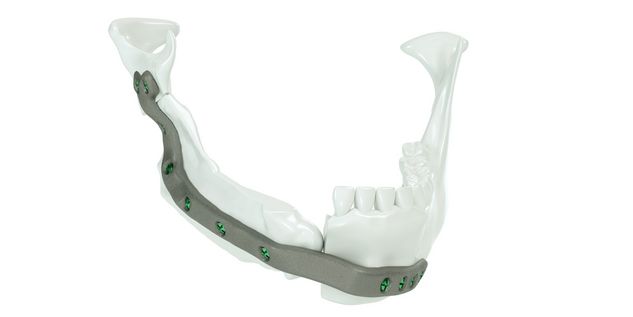
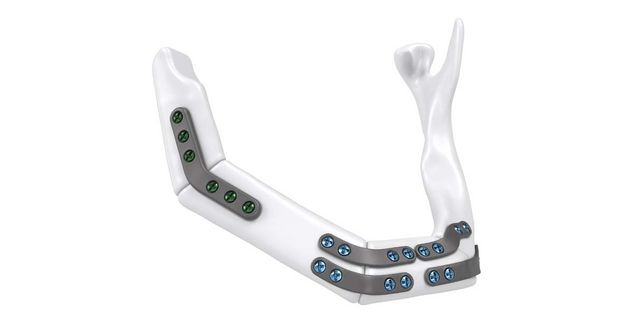
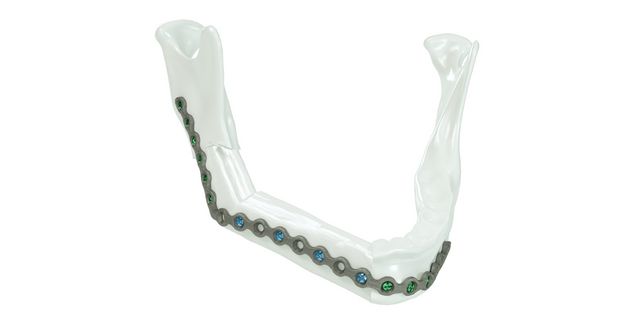
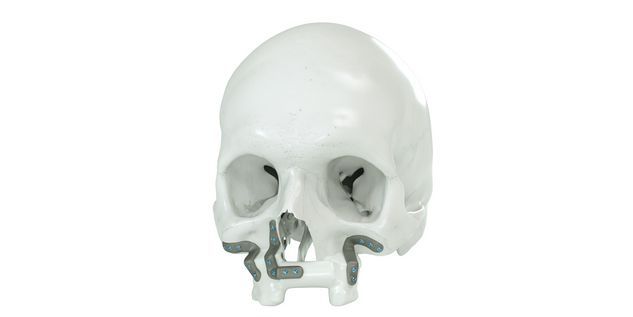
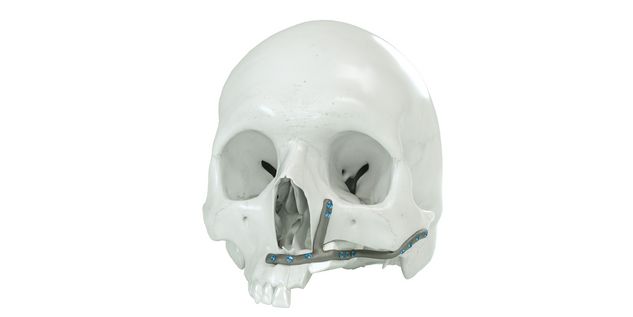
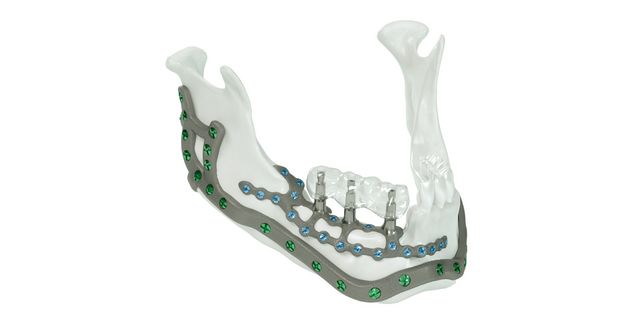
For the surgical restoration, the following osteosynthesis accessories in sterile condition are required for IPS Implants® Mandible Reconstruction:
According to the regulation (EU) 2017/745, a custom-made product is a device specifically made in accordance with a written prescription of any person authorized by national law by virtue of that person's professional qualifications which gives, under that person's responsibility, specific design characteristics, and is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.
For this reason a separate inquiry is necessary in every IPS® case.
The written prescription is a release for the technical offer (design of the products). This is to be submitted in writing by the user at the same time as the required order for the economic offer if he/she agrees with the desired case planning.
Shipment is not permissible as a matter of principle without this mandatory regulatory document.