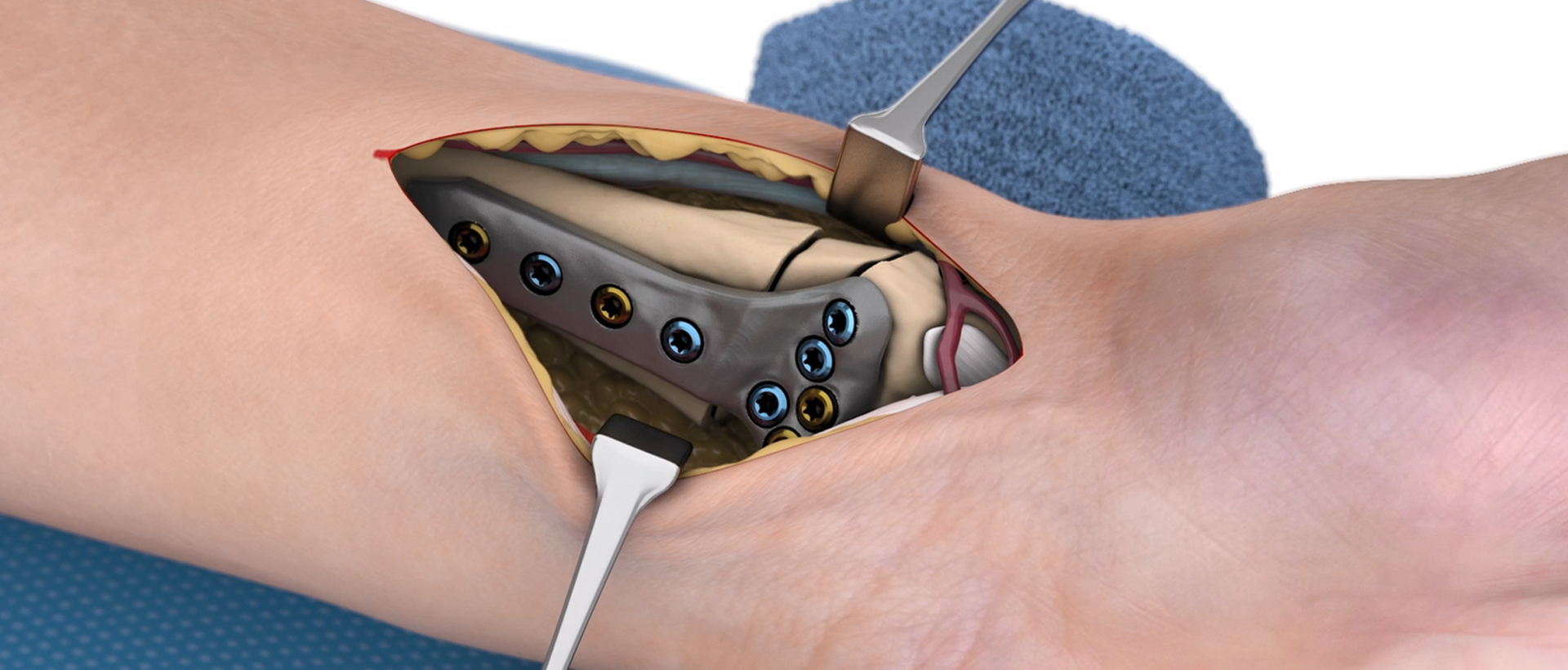
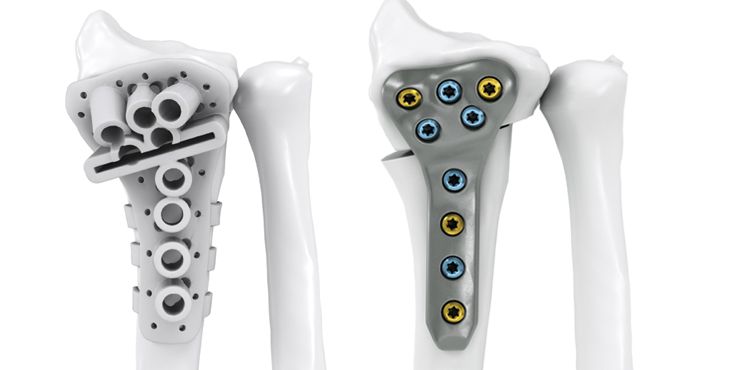
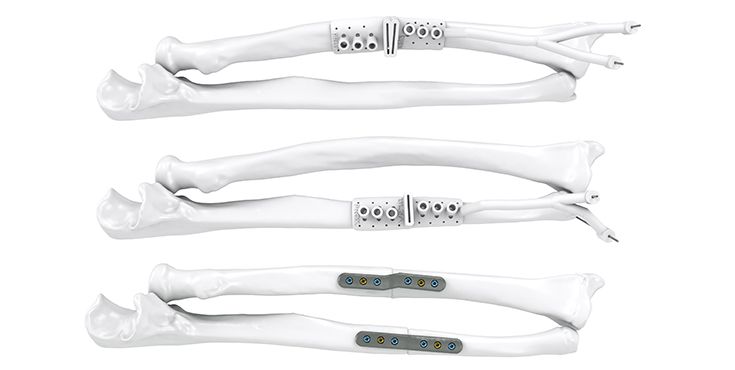
Dr. Jan-Ragnar Haugstvedt, Østfold Hospital, Moss, Norway: "After many years as a hand surgeon, one of the most challenging procedures to perform is correction of malunited forearm fractures that the patient suffered from in childhood. Using 3D reconstruction of the contralateral (non-injured) arm, and patient-specific guides and plates, the correction has been made “easy” with good results and happy patients."
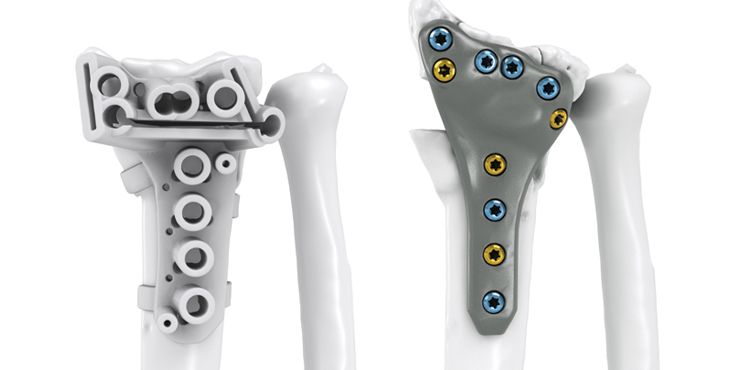
Dr. med. Philip Grieve, Blackrock Clinic, Dublin, Ireland: "With regards to the case in question, I simply wouldn’t have been able to treat this complex intra-articular malunion effectively without a patient specific implant and the 3D planning involved. On the day of surgery the support KLS Martin provided was top class and ensured the surgery was performed to the highest standard possible. I’m looking forward to my next case already."
Dr. med. Niels Schep, Maasstad Ziekenhuis Rotterdam, Netherlands: "IPS® provides me with the tools to plan and execute complex extra-articular malunions of the distal radius. Moreover, recently we also successfully completed intra-articular corrections with the help of patient-specific 3D-printed guides and titanium plates."
MUDr. Radek Kebrle, Vysoke nad Jizerou Hospital, Czech Republic: "3D planning and customized 3D printing in reconstructive surgery start to be inevitable solution for certain pathologies. Their use during reconstruction of intraarticular malunions of distal radius gives us a chance to reconstruct more complex cases that would be otherwise indicated for salvage."